initialize() {
// create a neutral mutation type
initializeMutationType("m1", 0.5, "f", 0.0);
// initialize 1Mb segment
initializeGenomicElementType("g1", m1, 1.0);
initializeGenomicElement(g1, 0, 999999);
// set mutation rate and recombination rate of the segment
initializeMutationRate(1e-8);
initializeRecombinationRate(1e-8);
}
// create an ancestral population p1 of 10000 diploid individuals
1 early() { sim.addSubpop("p1", 10000); }
// in generation 1000, create two daughter populations p2 and p3
1000 early() {
sim.addSubpopSplit("p2", 5000, p1);
sim.addSubpopSplit("p3", 1000, p1);
}
// in generation 10000, stop the simulation and save 100 individuals
// from p2 and p3 to a VCF file
10000 late() {
p2_subset = sample(p2.individuals, 100);
p3_subset = sample(p3.individuals, 100);
c(p2_subset, p3_subset).genomes.outputVCF("/tmp/slim_output.vcf.gz");
sim.simulationFinished();
catn("DONE!");
}slendr simulations
practical workshop at MPI EVA Leipzig
Many problems in population genetics cannot be solved by a mathematician, no matter how gifted. [It] is already clear that computer methods are very powerful. This is good. It […] permits people with limited mathematical knowledge to work on important problems […]
Why use simulations?
- Making sense of estimated statistics
- Fitting model parameters
- Ground truth for method work
Making sense of estimated statistics
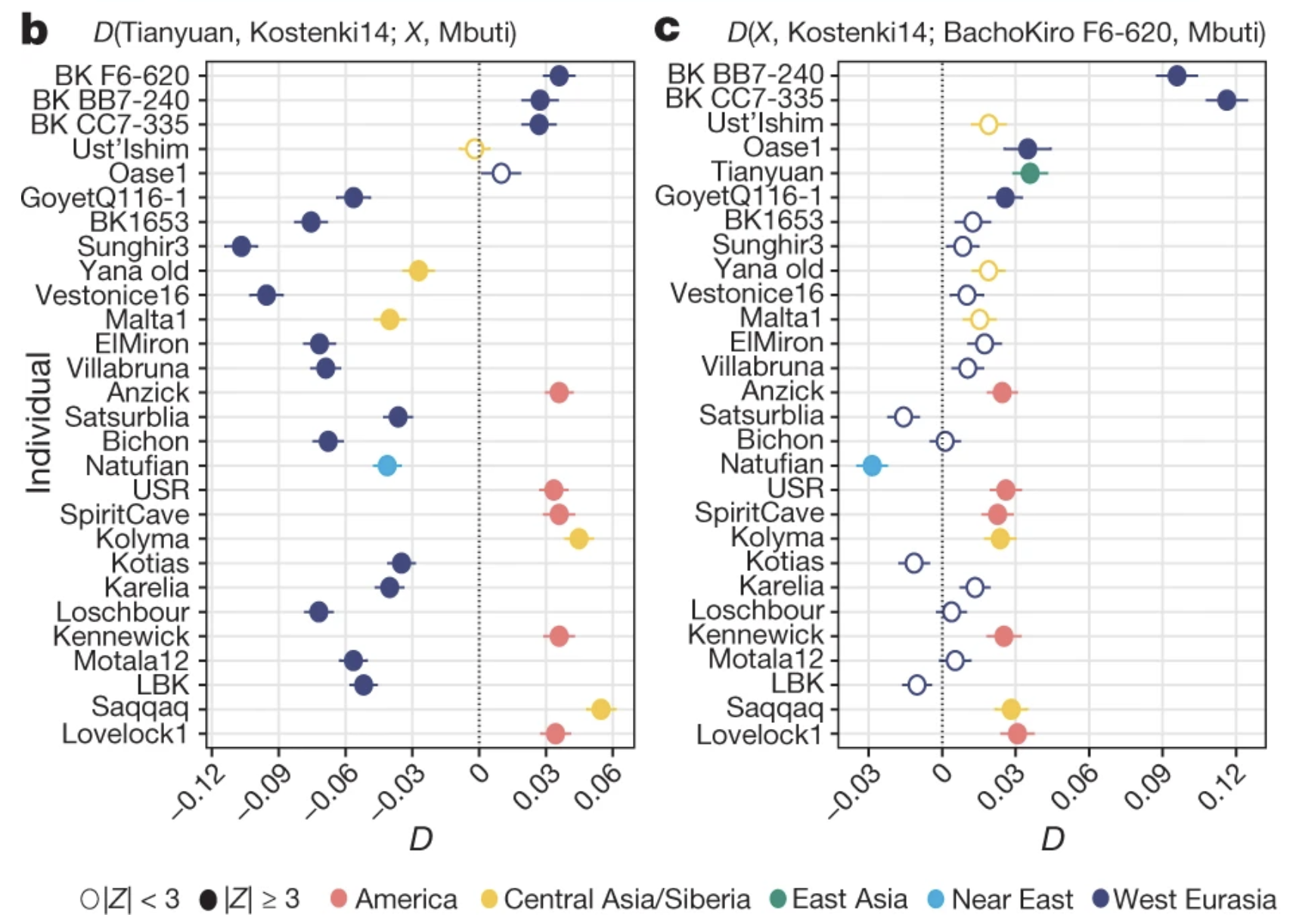
Making sense of estimated statistics
Making sense of estimated statistics
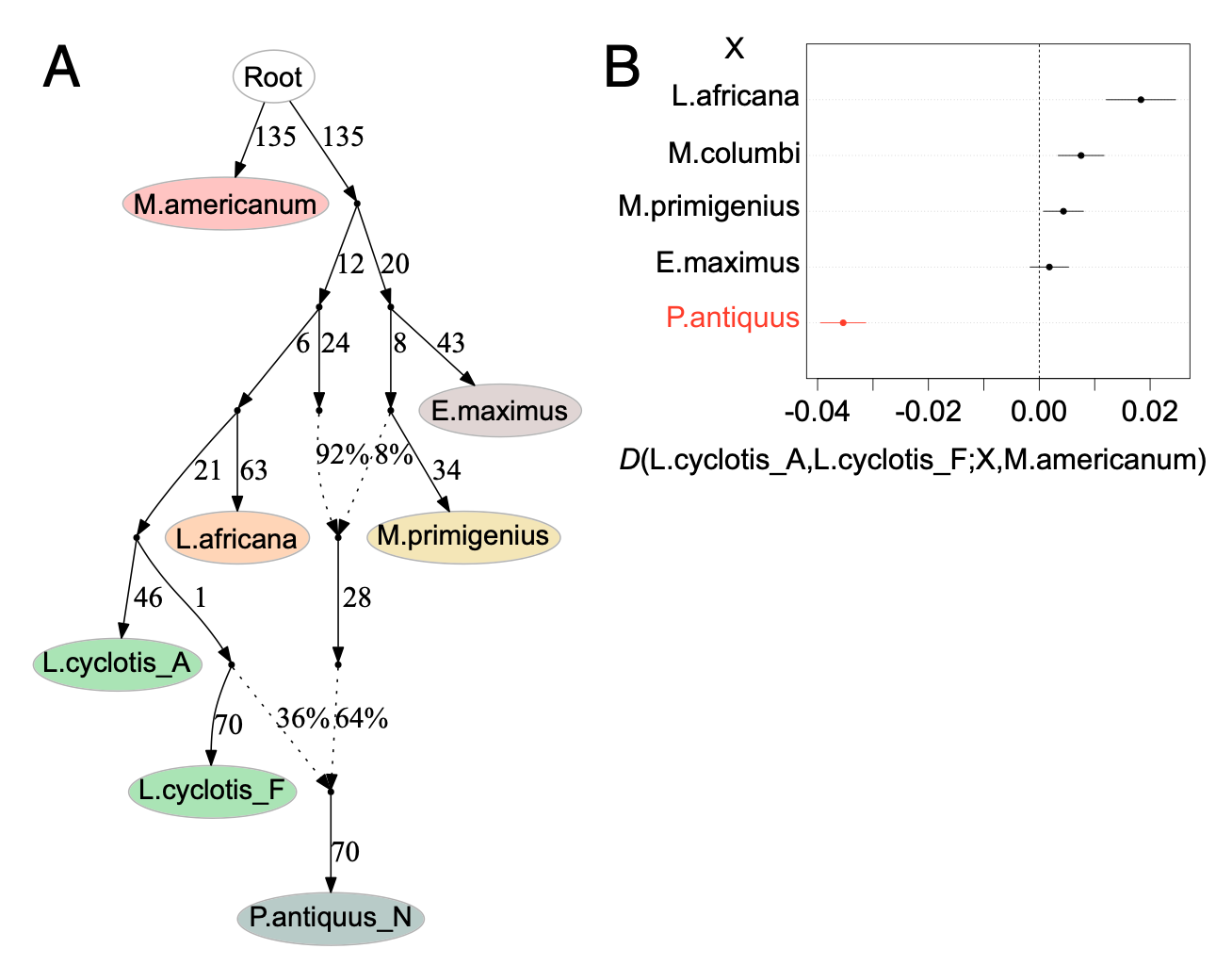
Fitting model parameters (i.e. ABC)
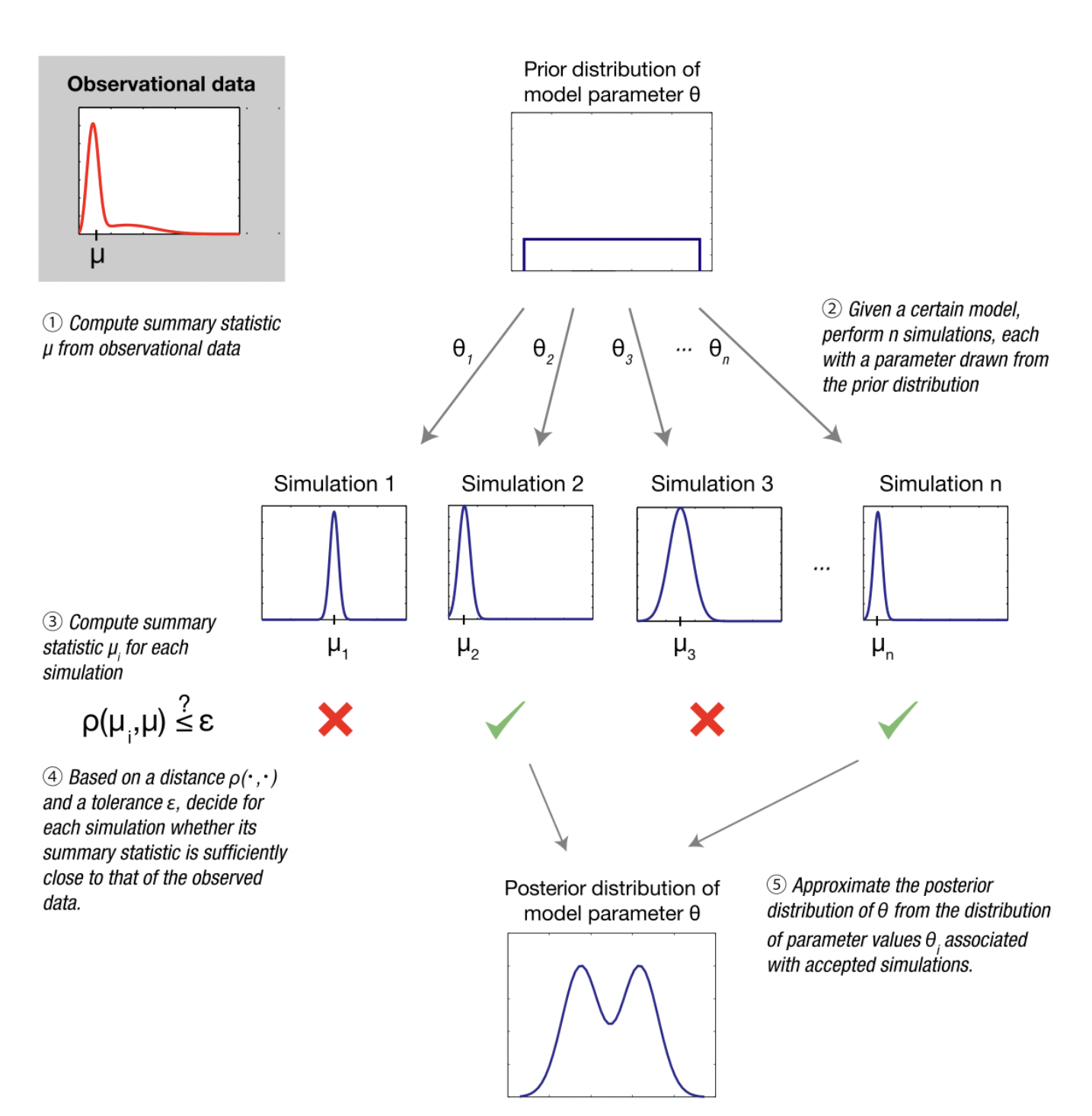
Image from Wikipedia on ABC
Ground truth for methods work

There are many simulation tools
The most famous and widely used are SLiM and msprime.
Both are very powerful…
… but they require a lot of programming knowledge...
… and a lot of code for non-trivial simulations (🐛🪲🐜).
a convenient R interface to both SLiM and msprime.
SLiM
What is SLiM?
- Forward-time simulator
- It’s fully programmable!
- Massive library of functions for:
- Demographic events
- Various mating systems
- Selection, quantitative traits, …
- > 700 pages long manual!
SLiMgui – IDE for SLiM

Simple neutral simulation in SLiM
msprime
What is msprime?

What is msprime?
- A Python module for writing coalescent simulations
- Extremely fast (genome-scale, population-scale data)
- You must know Python fairly well to build complex models
Simple simulation using msprime
This is basically the same model as the SLiM script earlier:
import msprime
demography = msprime.Demography()
demography.add_population(name="A", initial_size=10_000)
demography.add_population(name="B", initial_size=5_000)
demography.add_population(name="C", initial_size=1_000)
demography.add_population_split(time=1000, derived=["A", "B"], ancestral="C")
ts = msprime.sim_ancestry(
sequence_length=10e6,
recombination_rate=1e-8,
samples={"A": 100, "B": 100},
demography=demography
)source: link
www.slendr.net
Why a new package? – spatial simulations!

Why a new package?
Most researchers are not expert programmers
All but the most trivial simulations require lots of code
90%
of simulations are basically the same! create populations (splits and \(N_e\) changes)
specify if/how they should mix (rates and times)
save output (VCF, EIGENSTRAT)
- Lot of code duplication across projects
Let’s get started
We will need slendr & tidyverse
# load data analysis and plotting packages
library(dplyr)
Attaching package: 'dplyr'The following objects are masked from 'package:stats':
filter, lagThe following objects are masked from 'package:base':
intersect, setdiff, setequal, unionlibrary(ggplot2)
library(magrittr)
# load slendr itself
library(slendr)=======================================================================
NOTE: Due to Python setup issues on some systems which have been
causing trouble particularly for novice users, calling library(slendr)
no longer activates slendr's Python environment automatically.
In order to use slendr's msprime back end or its tree-sequence
functionality, users must now activate slendr's Python environment
manually by executing init_env() after calling library(slendr).
(This note will be removed in the next major version of slendr.)
=======================================================================
Attaching package: 'slendr'The following object is masked from 'package:magrittr':
subtractinit_env()The interface to all required Python modules has been activated.(ignore the message about missing SLiM)
slendr haiku
Build simple models,
simulate data from them.
Just one plain R script.
Typical steps (outline of this tutorial)
- creating populations
- scheduling population splits
- programming \(N_e\) size changes
- encoding gene-flow events
- simulation sequence of a given size
- computing statistics from simulated outputs
Creating a population()
Each needs a name, size and time of appearance (i.e., “split”):
pop1 <- population("pop1", N = 1000, time = 1)This creates a normal R object. Typing it gives a summary:
pop1slendr 'population' object
--------------------------
name: pop1
non-spatial population
stays until the end of the simulation
population history overview:
- time 1: created as an ancestral population (N = 1000)Programming population splits
Splits are indicated by the parent = <pop> argument:
pop2 <- population("pop2", N = 100, time = 50, parent = pop1)The split is reported in the “historical summary”:
pop2slendr 'population' object
--------------------------
name: pop2
non-spatial population
stays until the end of the simulation
population history overview:
- time 50: split from pop1 (N = 100)Scheduling resize events – resize()
Step size decrease:
Tidyverse-style pipe %>% interface
A more concise way to express the same thing as before.
Step size decrease:
pop1 <-
population("pop1", N = 1000, time = 1) %>%
resize(N = 100, time = 500, how = "step")Exponential increase:
pop2 <-
population("pop2", N = 1000, time = 1) %>%
resize(N = 10000, time = 500, end = 2000, how = "exponential")A more complex model
pop1 <- population("pop1", N = 1000, time = 1)
pop2 <-
population("pop2", N = 1000, time = 300, parent = pop1) %>%
resize(N = 100, how = "step", time = 1000)
pop3 <-
population("pop3", N = 1000, time = 400, parent = pop2) %>%
resize(N = 2500, how = "step", time = 800)
pop4 <-
population("pop4", N = 1500, time = 500, parent = pop3) %>%
resize(N = 700, how = "exponential", time = 1200, end = 2000)
pop5 <-
population("pop5", N = 100, time = 600, parent = pop4) %>%
resize(N = 50, how = "step", time = 900) %>%
resize(N = 250, how = "step", time = 1200) %>%
resize(N = 1000, how = "exponential", time = 1600, end = 2200) %>%
resize(N = 400, how = "step", time = 2400)Remember that slendr objects internally carry their whole history!
pop5slendr 'population' object
--------------------------
name: pop5
non-spatial population
stays until the end of the simulation
population history overview:
- time 600: split from pop4 (N = 100)
- time 900: resize from 100 to 50 individuals
- time 1200: resize from 50 to 250 individuals
- time 1600-2200: exponential resize from 250 to 1000 individuals
- time 2400: resize from 1000 to 400 individualsLast step before simulation: compile_model()
model <- compile_model(
list(pop1, pop2, pop3, pop4, pop5),
generation_time = 1,
simulation_length = 3000
)The model is also compiled to disk which gives a nice additional layer of reproducibility. The exact location can be specified via path = argument to compile_model().
Model summary
Typing the compiled model into R prints a brief summary:
modelslendr 'model' object
---------------------
populations: pop1, pop2, pop3, pop4, pop5
geneflow events: [no geneflow]
generation time: 1
time direction: forward
total running length: 3000 model time units
model type: non-spatial
configuration files in: /private/var/folders/d_/hblb15pd3b94rg0v35920wd80000gn/T/Rtmp8Mdden/filed642a032e2 Model visualization
plot_model(model)
Exercise #1
Exercise #1 — write your own model!
You can use this “template”:
library(slendr)
init_env()
chimp <- population(...)
# <... rest of your code ...>
model <- compile_model(
populations = list(chimp, ...),
generation_time = 30
)
plot_model(model) # verify visually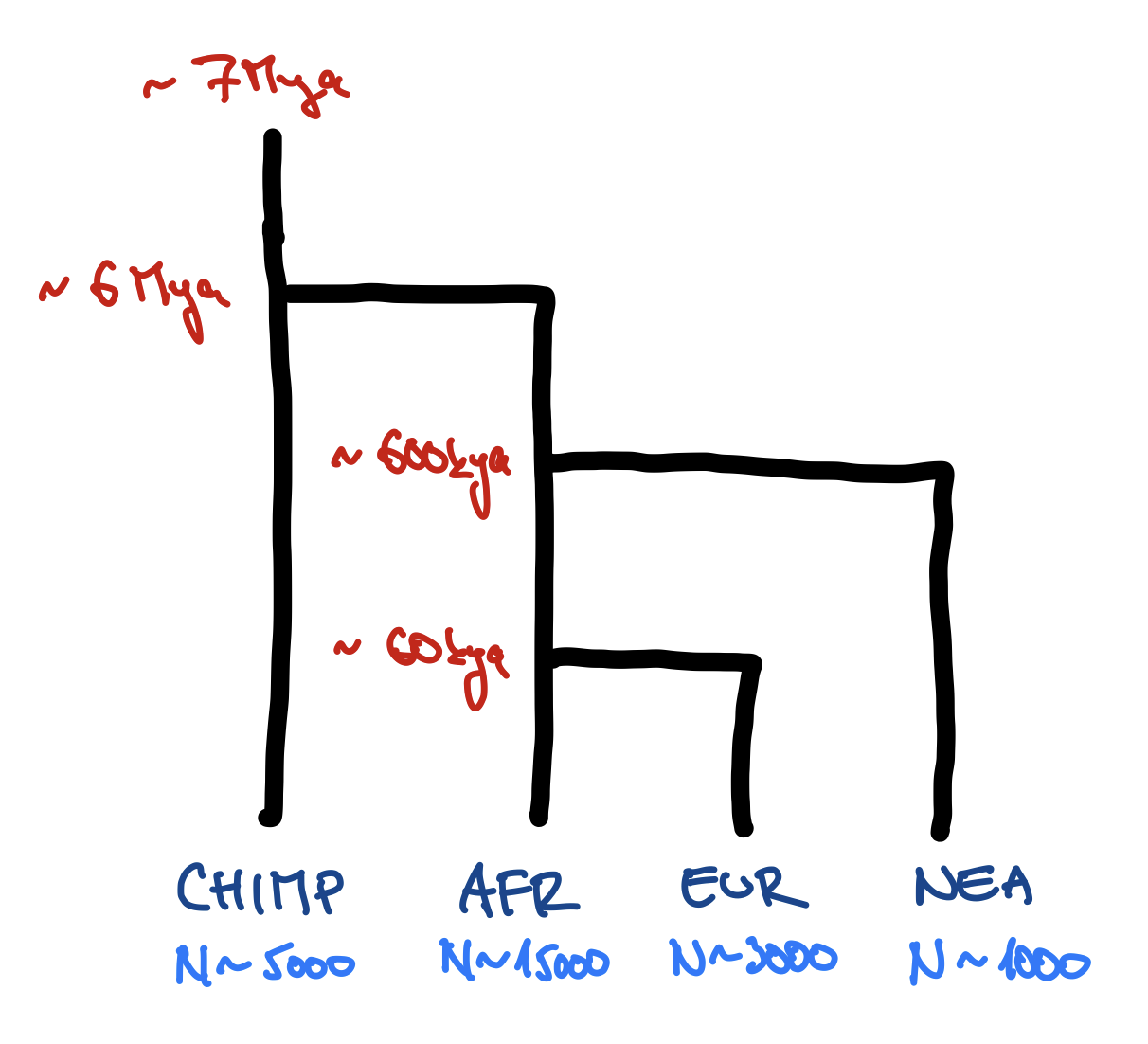
Don’t worry about gene flow just yet. We will add that at a later stage.
Feel free to include expansions and contractions (maybe in EUR at some point?).
Exercise #1 — solution
Simulating data (finally…)
We have a compiled model, how do we simulate data?
slendr has two built-in simulation engines:
- SLiM engine
- msprime engine
You don’t have to write any msprime or SLiM code!
This is all that’s needed:
ts <- msprime(model, sequence_length = 100e6, recombination_rate = 1e-8)ts is a so-called tree sequence)
The output of a slendr simulation is a tree sequence
What is tree sequence?
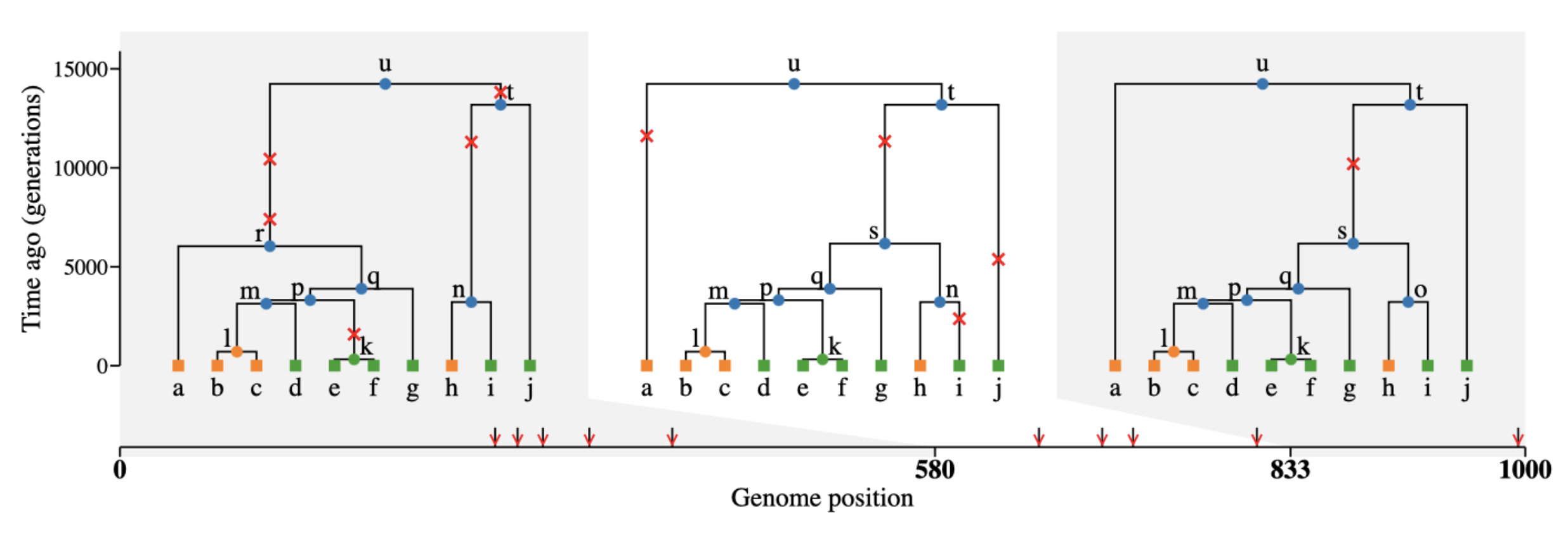
- a record of full genetic ancestry of a set of samples
- an encoding of DNA sequence carried by those samples
- an efficient analysis framework
Why tree sequence?
Why not VCF, EIGENSTRAT, or a genotype table?
What we usually have
What we usually want
(As full as possible) a representation of our samples’ history:

Here is the magic
Tree sequences make it possible to directly compute many quantities of interest without going via conversion to a genotype table/VCF!

How can we compute statistics without mutations?
There is a duality between mutations and branch lengths in trees (more here).

But what if we want mutations?
Coalescent and mutation processes can be decoupled

This means we can add mutations
after the simulation.
This allows efficient, massive simulations
If we have a simulated ts object, we can do:
ts_mutated <- ts_mutate(ts, mutation_rate = 1e-8)
Or, with a shortcut:
ts <-
msprime(model, sequence_length = 100e6, recombination_rate = 1e-8) %>%
ts_mutate(mutation_rate = 1e-8 )ts_mutate() throughout.
Tree sequences are also very efficient
Let’s take this minimalistic model:
pop <- population("pop", time = 1e6, N = 10000)
model <- compile_model(pop, generation_time = 30, direction = "backward")
ts <- msprime(model, sequence_length = 1e6, recombination_rate = 1e-8)(simulates 2 \(\times\) 10000 chromosomes of 100 Mb)
Runs in less than 30 seconds on my laptop!
Taking about 66 Mb of memory!
How does this work?!

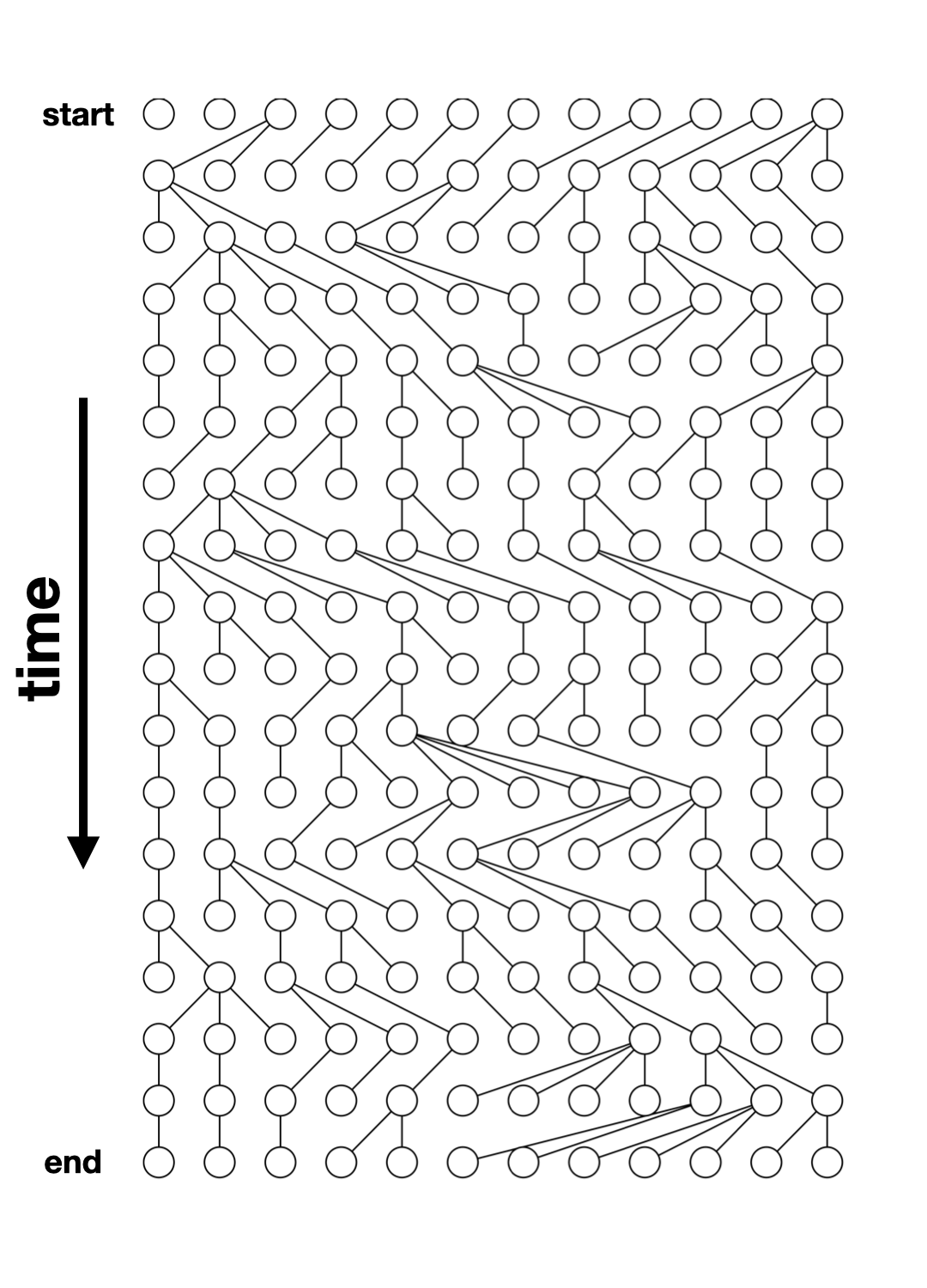
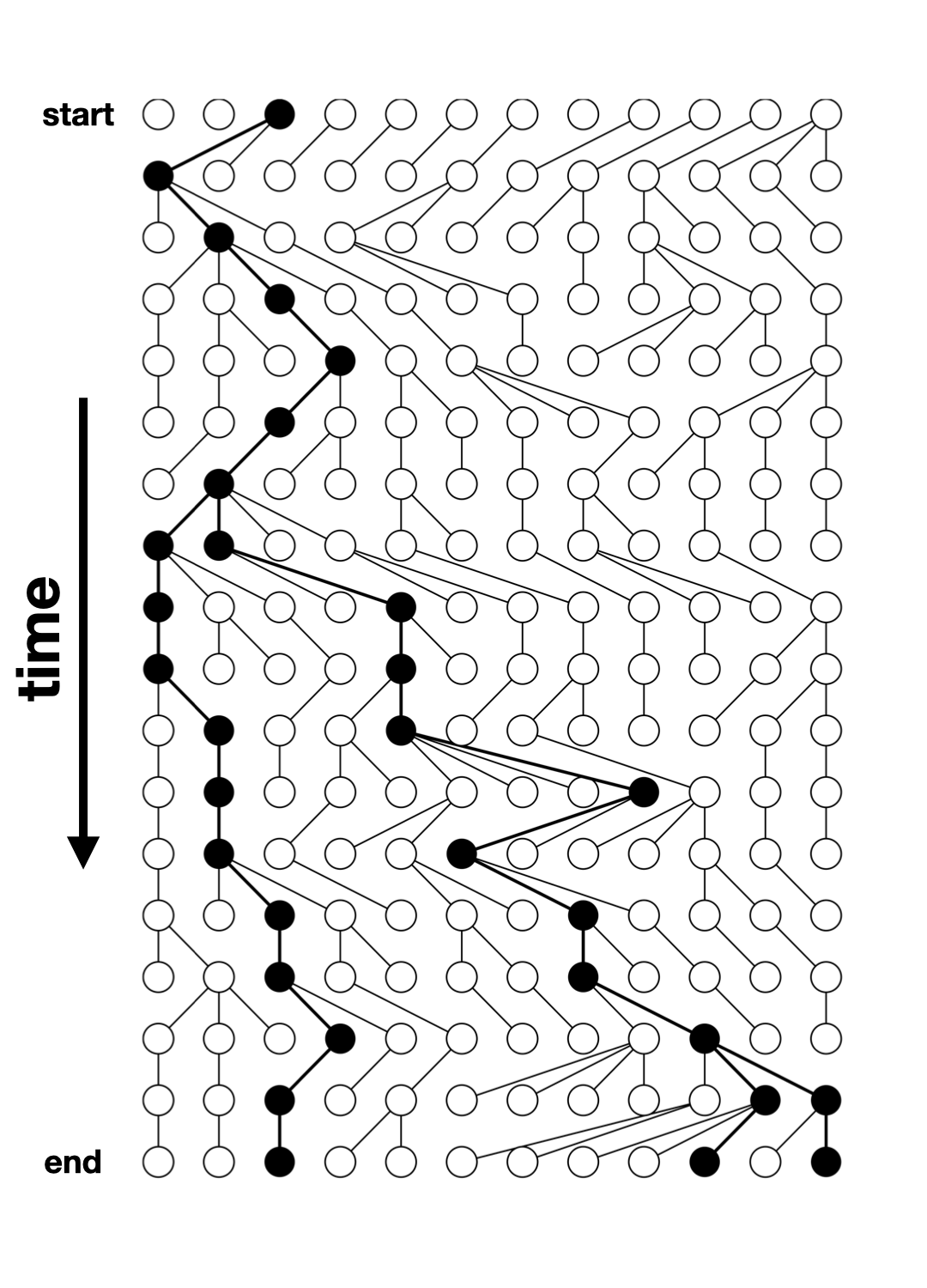
Tree-sequence tables (tskit docs)
A tree (sequence) can be represented by
- a table of nodes,
- a table of edges between nodes,
- a table of mutations on edges

A set of such tables is a tree sequence.
Tree-sequence tables in practice
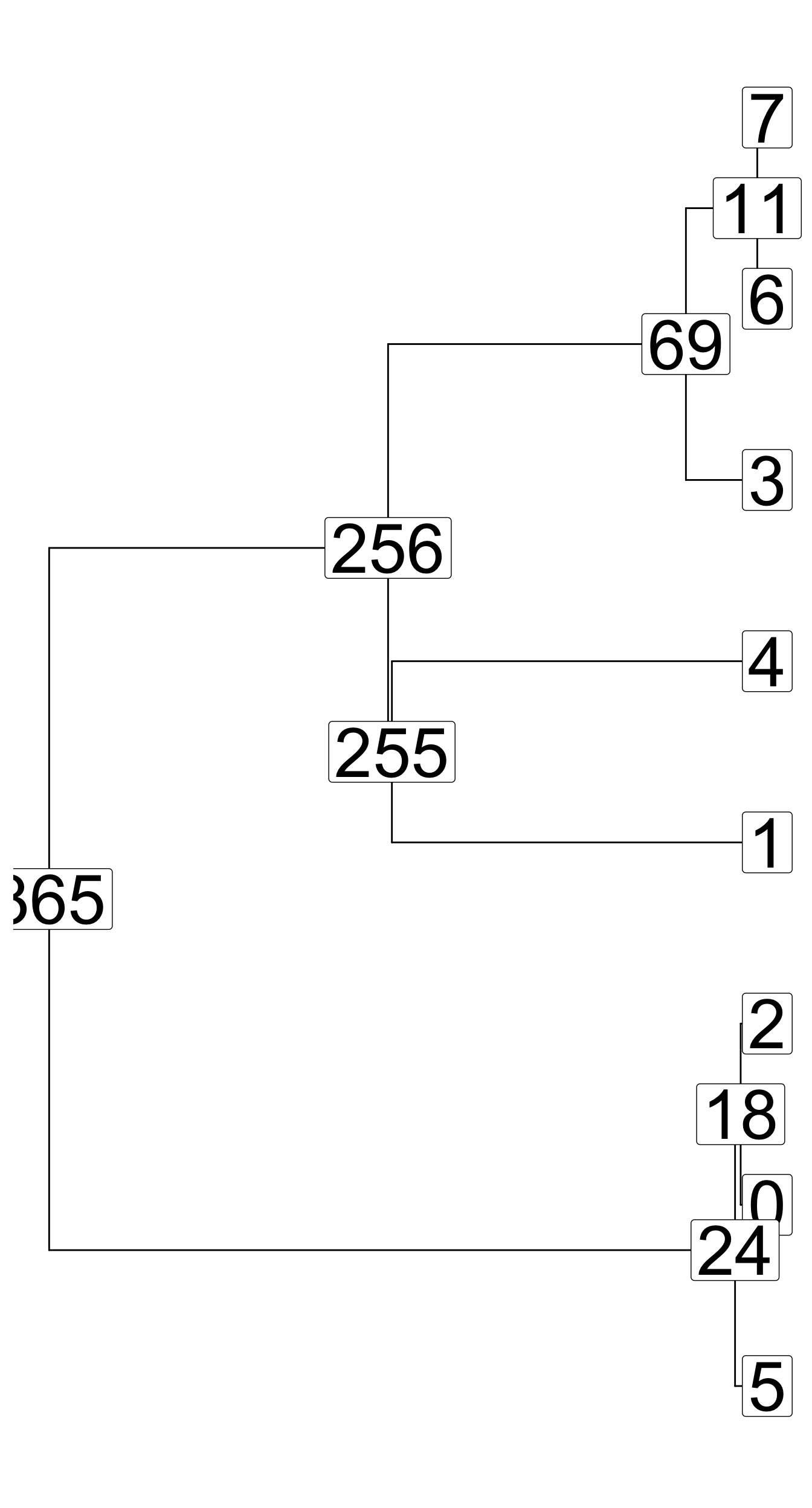
nodes:
node_id pop_id time
1 365 0 901433.1
2 256 0 475982.3
3 255 0 471179.5edges:
child_node_id parent_node_id
1 256 365
2 255 256
3 69 256mutations:
[1] id site node time
<0 rows> (or 0-length row.names)Let’s take the model we defined earlier…
… and simulate tree sequence from it
ts <- msprime(model, sequence_length = 1e6, recombination_rate = 1e-8)ts_save().)
Conversion to other genotype formats
If you have a tree-sequence object ts, you can do…
ts_vcf(ts, path = "path/to/a/file.vcf.gz")ts_eigenstrat(ts, prefix = "path/to/eigenstrat/prefix")ts_genotypes(ts)# A tibble: 935 × 7
pos pop_1_chr1 pop_1_chr2 pop_2_chr1 pop_2_chr2 pop_3_chr1 pop_3_chr2
<int> <int> <int> <int> <int> <int> <int>
1 670 0 0 0 0 0 1
2 790 0 0 0 1 0 0
3 3525 1 0 1 0 0 0
4 4224 0 1 0 0 1 0
5 7036 1 0 1 0 0 0
6 8019 1 0 1 0 0 0
7 9063 0 1 0 1 1 1
8 9961 1 1 1 0 1 0
9 10623 0 0 0 1 0 1
10 12526 1 1 1 0 1 0
# … with 925 more rowsWhat can we do with it?
slendr’s R interface to tskit

This R interface links to Python methods implemented in tskit.
Extracting sample information
Each “sampled” individual in slendr has a symbolic name, a sampling time, and a population assignment.

Extracting sample information
If we have a tree sequence ts, we can get samples with ts_samples():
ts_samples(ts)# A tibble: 3 × 3
name time pop
<chr> <dbl> <chr>
1 pop_1 0 pop
2 pop_2 0 pop
3 pop_3 0 pop
ts_samples(ts) %>% count(pop)# A tibble: 1 × 2
pop n
<chr> <int>
1 pop 3Analyzing tree sequences with slendr
Let’s say we have the following model and we simulate a tree sequence from it.
pop <- population("pop", N = 10000, time = 1)
model <- compile_model(pop, generation_time = 1, simulation_length = 10000)
ts <-
msprime(model, sequence_length = 100e6, recombination_rate = 1e-8) %>%
ts_mutate(mutation_rate = 1e-8)Example: allele frequency spectrum
# sample 5 individuals
samples <-
ts_samples(ts) %>%
sample_n(5) %>%
pull(name)
samples[1] "pop_2369" "pop_5273" "pop_9290" "pop_1252" "pop_8826"# compute the AFS
afs <- ts_afs(
ts, list(samples),
polarised = TRUE
)
afs [1] 39887 19809 13465 10387 8024 6634 5543 5134 4567 3888
plot(afs, type = "b",
xlab = "allele count bin",
ylab = "frequency")
Exercise #2
Exercise #2 — estimating \(N_e\) from AFS
You sequenced 10 individuals from one population and computed this AFS (counts of singletons, doubletons, …):
afs_observed <- c(2520, 1449, 855, 622, 530, 446, 365, 334, 349, 244,
264, 218, 133, 173, 159, 142, 167, 129, 125, 143)You know from fossil evidence that the population had constant \(N_e\) for the past 100,000 generations, and that the \(N_e\) was somewhere between 1000 and 30000.
Use slendr to guess the true value of \(N_e\) given the observed AFS by running single-population simulations of different \(N_e\) and comparing ts_afs() results to afs_observed.
Exercise #2 – hints
Write an R function (in a new script) that gets \(N_e\) as input, creates a slendr population, compiles a model, simulates a tree sequence, runs
ts_afs()on it, and returns the AFS.Find the \(N_e\) value that will give the closest AFS to the observed one. For instance, you could:
a ) Plot simulated AFS for different \(N_e\) with the AFS and just eyeball \(N_e\) value that looks correct from a graph.
b ) Simulate AFS automatically in steps of possible \(N_e\) values and find the closest matching one.
Exercise #2 solution (a) – eye-balling
Exercise #2 solution (b) – grid
Gene flow / admixture
gene_flow() events
Gene flow is programmed the gene_flow() function.
If we have populations p1 and p2, we schedule gene flow with:
gf <- gene_flow(from = p1, to = p2, start = 500, end = 600, rate = 0.13)Multiple gene-flow events can be gathered in a list:
gf <- list(
gene_flow(from = p1, to = p2, start = 500, end = 600, rate = 0.13),
gene_flow(from = <..>, to = <..>, start = <...>, end = <...>, rate = <...>),
< potentially many more ... >
)gene_flow() checks for consistency!
Plugging gene flow(s) into the model
gf <- gene_flow(from = ..., to = ..., <start, end, rate>)
model <- compile_model(
populations = list(...),
gene_flow = gf, # <--- gene flow(s) specified
generation_time = 30
)Exercise #3
Exercise #3 — adding gene_flow()
Add Neanderthal introgression into your model from Exercise #1 (let’s say 3% pulse NEA -> EUR at 55 kya).
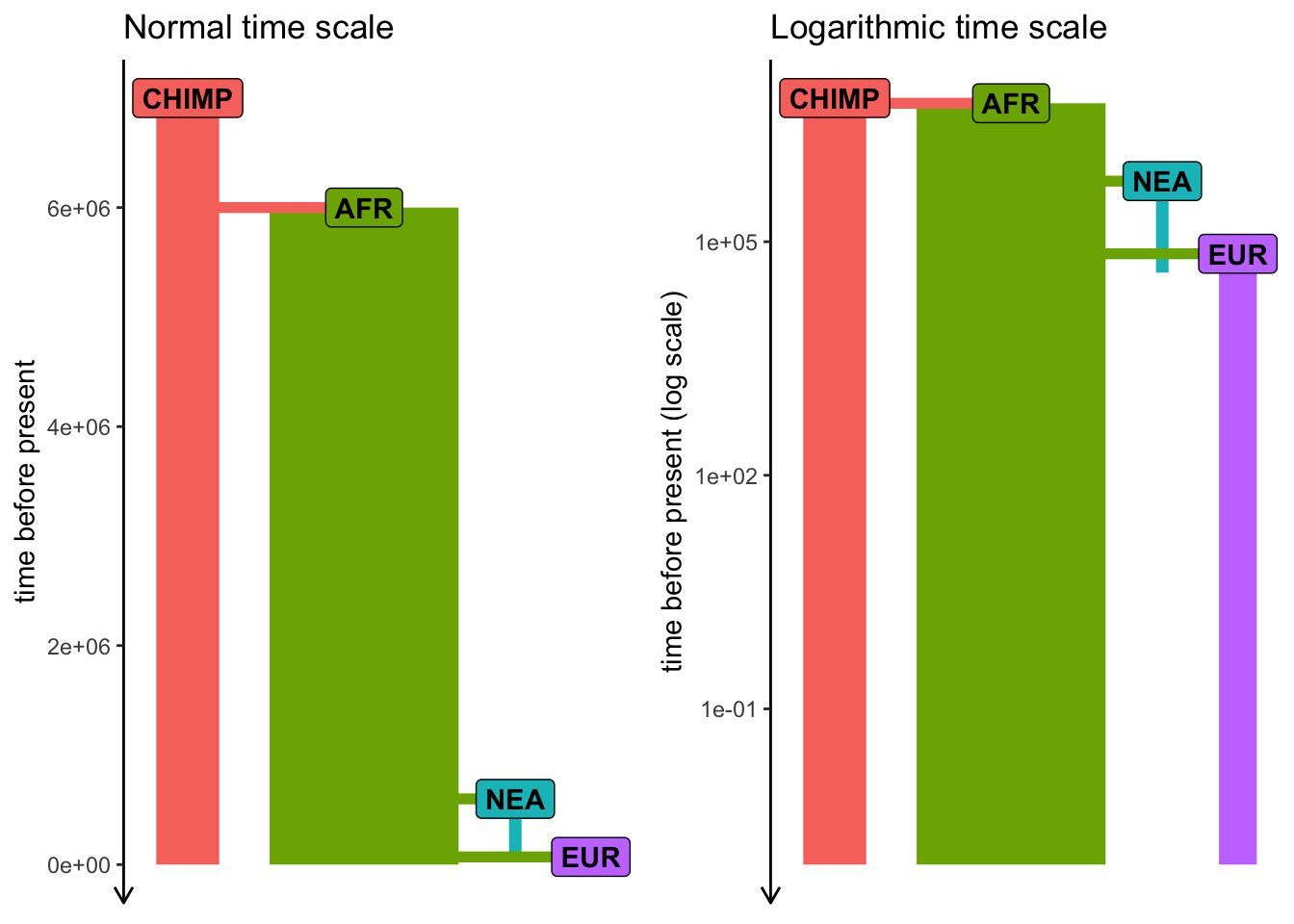
Exercise #3 — adding gene_flow()
Add Neanderthal introgression into your model from Exercise #1 (let’s say 3% pulse NEA -> EUR at 55 kya).
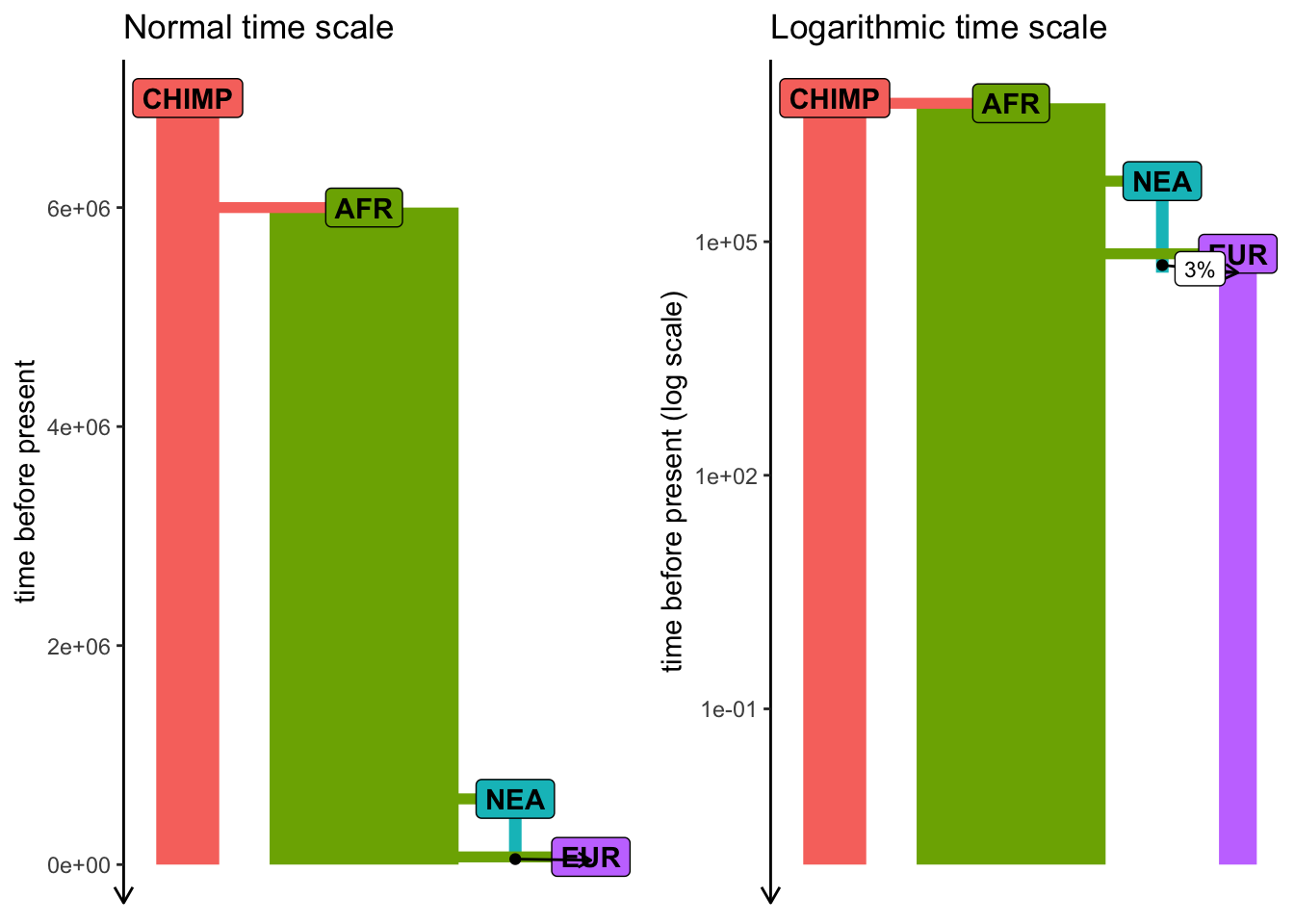
Exercise #3 — solution
Exercise #4
Exercise #4 — popgen statistics
plot_model(model)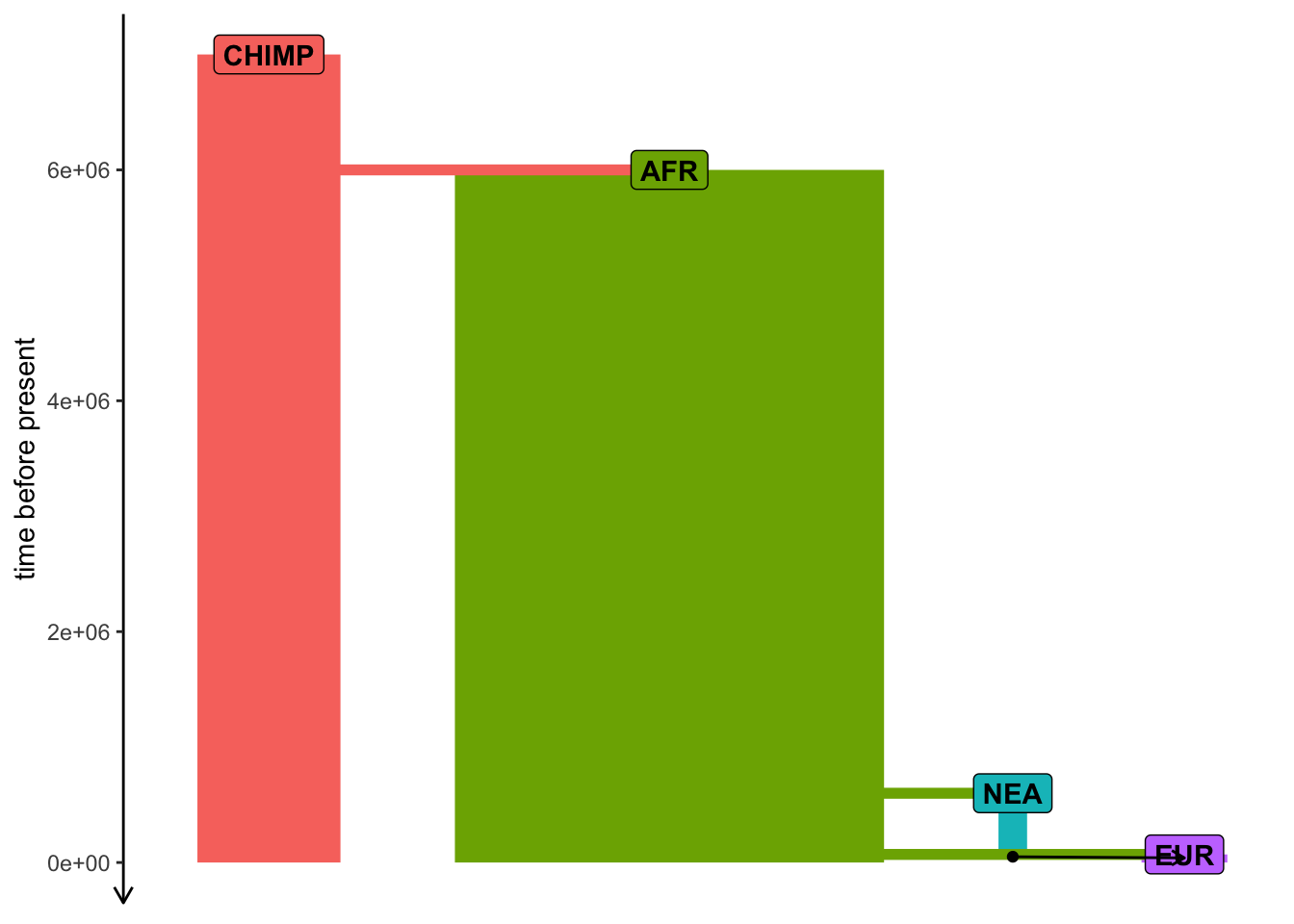
Exercise #4 — popgen statistics
Compute nucleotide diversity (\(\pi\)) in each population (ts_diversity()). Do your results with known \(N_e\) values?
Compute ts_divergence() (genetic divergence) or ts_fst() (\(F_{ST}\)) between populations. Do the results match the model?
Compute the “outgroup \(f_3\)” using CHIMP as the outgroup (ts_f3()) for different pairs of “A” and “B” populations.
Test Neanderthal introgression in EUR using the \(f_4\) statistic (ts_f4()). [hint: \(f_4\)(AFR, EUR; NEAND, CHIMP)]
Exercise #4 — popgen statistics
A useful R trick for handling recorded samples:
ts_samples(ts)# A tibble: 24,000 × 3
name time pop
<chr> <dbl> <chr>
1 AFR_1 0 AFR
2 AFR_2 0 AFR
3 AFR_3 0 AFR
4 AFR_4 0 AFR
5 AFR_5 0 AFR
6 AFR_6 0 AFR
7 AFR_7 0 AFR
8 AFR_8 0 AFR
9 AFR_9 0 AFR
10 AFR_10 0 AFR
# … with 23,990 more rowssamples <- ts_samples(ts) %>%
split(., .$pop) %>%
lapply(pull, "name")str(samples)List of 4
$ AFR : chr [1:15000] "AFR_1" "AFR_2" "AFR_3" "AFR_4" ...
$ CHIMP: chr [1:5000] "CHIMP_1" "CHIMP_2" "CHIMP_3" "CHIMP_4" ...
$ EUR : chr [1:3000] "EUR_1" "EUR_2" "EUR_3" "EUR_4" ...
$ NEA : chr [1:1000] "NEA_1" "NEA_2" "NEA_3" "NEA_4" ...Exercise #4 — solution
Time-series data
Sampling aDNA samples through time
Imagine we have pop1, pop2, … compiled in a model.
To record ancient individuals in the tree sequence, we can use schedule_sampling() like this:
schedule_sampling(
model, # compiled slendr model object
times = c(100, 500), # at these times (can be also a single number) ...
list(pop1, 42), # ... sample 42 individuals from pop1
list(pop2, 10), # ... sample 10 individuals from pop2
list(pop3, 1) # ... sample 1 individual from pop 3
) Sampling schedule format
The output of schedule_sampling() is a plain data frame:
schedule_sampling(model, times = c(40000, 30000, 20000, 10000), list(eur, 1))# A tibble: 4 × 7
time pop n y_orig x_orig y x
<int> <chr> <int> <lgl> <lgl> <lgl> <lgl>
1 10000 EUR 1 NA NA NA NA
2 20000 EUR 1 NA NA NA NA
3 30000 EUR 1 NA NA NA NA
4 40000 EUR 1 NA NA NA NA We can bind multiple sampling schedules together, giving us finer control about sampling:
eur_samples <- schedule_sampling(model, times = c(40000, 30000, 20000, 10000, 0), list(eur, 1))
afr_samples <- schedule_sampling(model, times = 0, list(afr, 1))
samples <- bind_rows(eur_samples, afr_samples)How to use a sampling schedule?
To sample individuals based on a given schedule, we use the samples = argument of the msprime() functions:
ts <- msprime(model, samples = samples, sequence_length = 1e6, recombination_rate = 1e-8) %>%
ts_mutate(mutation_rate = 1e-8)We can verify that only specific individuals are recorded:
ts_samples(ts)# A tibble: 6 × 3
name time pop
<chr> <dbl> <chr>
1 EUR_1 40000 EUR
2 EUR_2 30000 EUR
3 EUR_3 20000 EUR
4 EUR_4 10000 EUR
5 AFR_1 0 AFR
6 EUR_5 0 EUR ts╔═══════════════════════════╗
║TreeSequence ║
╠═══════════════╤═══════════╣
║Trees │ 1189║
╟───────────────┼───────────╢
║Sequence Length│ 1000000║
╟───────────────┼───────────╢
║Time Units │generations║
╟───────────────┼───────────╢
║Sample Nodes │ 12║
╟───────────────┼───────────╢
║Total Size │ 264.9 KiB║
╚═══════════════╧═══════════╝
╔═══════════╤════╤═════════╤════════════╗
║Table │Rows│Size │Has Metadata║
╠═══════════╪════╪═════════╪════════════╣
║Edges │3616│113.0 KiB│ No║
╟───────────┼────┼─────────┼────────────╢
║Individuals│ 6│192 Bytes│ No║
╟───────────┼────┼─────────┼────────────╢
║Migrations │ 0│ 8 Bytes│ No║
╟───────────┼────┼─────────┼────────────╢
║Mutations │1580│ 57.1 KiB│ No║
╟───────────┼────┼─────────┼────────────╢
║Nodes │ 858│ 23.5 KiB│ No║
╟───────────┼────┼─────────┼────────────╢
║Populations│ 4│341 Bytes│ Yes║
╟───────────┼────┼─────────┼────────────╢
║Provenances│ 2│ 3.5 KiB│ No║
╟───────────┼────┼─────────┼────────────╢
║Sites │1579│ 38.6 KiB│ No║
╚═══════════╧════╧═════════╧════════════╝Exercise #5
Exercise #5a — ancient samples
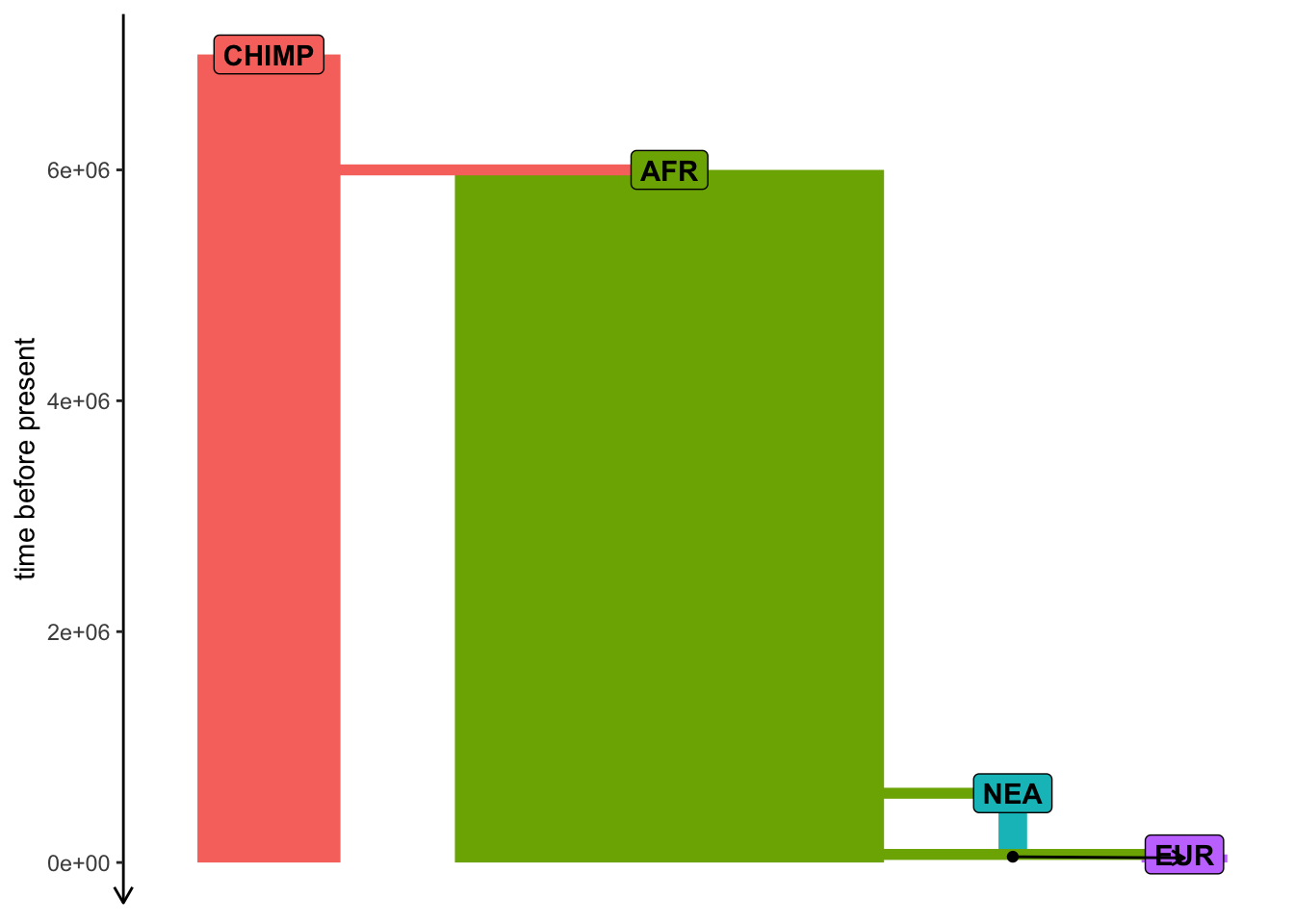
Exercise #5a — ancient samples
Simulate data from your model using this sampling:
- one present-day CHIMP and AFR individual
- 20 present-day EUR individuals
- 1 NEA at 70 ky, 1 NEA at 40 ky
- 1 EUR every 1000 years between 50-5 kya
Reminder: you can do this by:
samples <- # rbind(...) together individual schedule_sampling() data frames
ts <-
msprime(model, samples = samples, sequence_length = 100e6, recombination_rate = 1e-8) %>%
ts_mutate(mutation_rate = 1e-8)Exercise #5b — \(f_4\)-ratio statistic
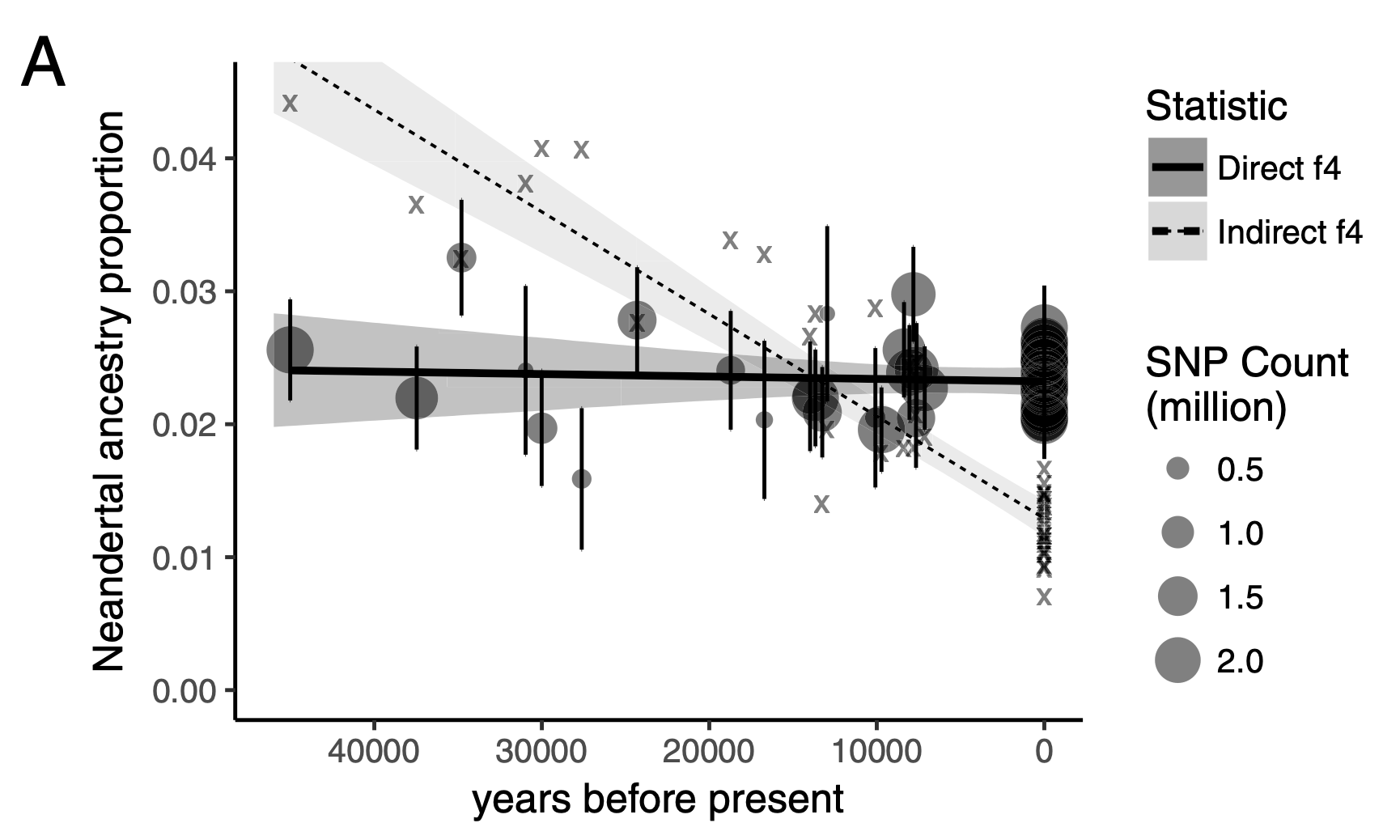
Use \(f_4\)-ratio statistic to replicate the following figure:

Hint: You can compute Neanderthal ancestry for a vector of individuals X as ts_f4ratio(ts, X = X, "NEA_1", "NEA_2", "AFR_1", "CHIMP_1").
Exercise #5 — solution
More information
slendr paper on bioRxiv
Documentation, tutorials can be found here
GitHub repo (bug reports!) is here
- new R package for ABC modeling demografr
